Chronic Kidney Disease (CKD) is a prevalent health issue in older cats, significantly impacting their well-being and lifespan. Managing CKD often involves various treatments aimed at supporting kidney function and reducing the build-up of toxins in the blood, a condition known as azotemia. Probiotics, and specifically synbiotics like Azodyl, have gained attention as a potential supplementary therapy to help manage azotemia in cats with CKD. Azodyl is designed to support kidney function by utilizing beneficial bacteria to break down waste products in the gut, effectively acting as a form of “enteric dialysis.” While the manufacturer recommends administering Azodyl capsules intact, many cat owners find this challenging and opt to sprinkle the capsule contents onto their cat’s food or mix it into a slurry. This raises a crucial question: is Azodyl effective when administered in this non-recommended manner? This article will delve into a study that investigated the effectiveness of Azodyl For Cats with CKD when given by sprinkling it on food, providing valuable insights for cat owners and veterinarians alike.
Understanding Chronic Kidney Disease and Azodyl
Chronic Kidney Disease in cats is a progressive condition where the kidneys gradually lose their ability to filter waste products from the blood effectively. This leads to a build-up of toxins, including urea and creatinine, resulting in azotemia. Azodyl is a synbiotic, combining probiotics and prebiotics, formulated to aid in reducing azotemia in cats with CKD. It contains specific strains of bacteria – Streptococcus thermophilus, Lactobacillus acidophilus, and Bifidobacterium longum – along with psyllium husk as a prebiotic. These bacteria are intended to metabolize uremic toxins in the intestines, reducing the burden on the kidneys. Azodyl is designed to be delivered in enteric-coated capsules to protect the bacteria from stomach acid and release them in the lower intestinal tract for optimal efficacy.
However, administering capsules to cats can be challenging. Many owners resort to opening the capsules and mixing the contents with food to ensure their cats consume the supplement. This practice deviates from the manufacturer’s recommendation and could potentially compromise Azodyl’s effectiveness. The stomach acid may degrade the bacteria before they reach the intestines, and the anaerobic bacterium Bifidobacterium longum might become inactive if exposed to air before reaching its intended site of action in the distal intestine.
Recognizing this common practice and the lack of scientific data supporting the efficacy of non-recommended administration, a study was conducted to evaluate whether Azodyl reduces azotemia in cats with CKD when sprinkled onto food or given as a slurry.
Study Design: Evaluating Non-Recommended Azodyl Administration
To determine the effectiveness of Azodyl when administered by sprinkling onto food, a controlled, double-blinded clinical trial was conducted. Ten cats diagnosed with stable CKD were enrolled in the study. The cats were randomly assigned to one of two groups: one group received Azodyl (synbiotic group), and the other received psyllium husk only (placebo group). Psyllium husk was chosen as the placebo because it is the prebiotic component and filler in Azodyl. Both owners and veterinarians involved were unaware of which treatment each cat received, ensuring objectivity in the study.
Prior to starting the treatment, the cats’ blood urea nitrogen (BUN) and creatinine levels were measured twice to establish a stable baseline for their kidney function. During the two-month treatment period, BUN and creatinine levels were measured monthly. Owners were instructed to administer the assigned treatment by sprinkling the capsule contents onto the cat’s food or mixing it with a small amount of food or water to create a slurry. Administering the capsules intact was not allowed in this study to mimic the common non-recommended practice.
To assess the impact of each treatment, the maximal percentage change in BUN and creatinine levels was calculated for each cat, comparing their highest baseline values to their lowest values during the treatment period. This approach aimed to maximize the chance of detecting any potential effect of Azodyl. Statistical analysis was then performed to compare the percentage changes in BUN and creatinine between the Azodyl and placebo groups.
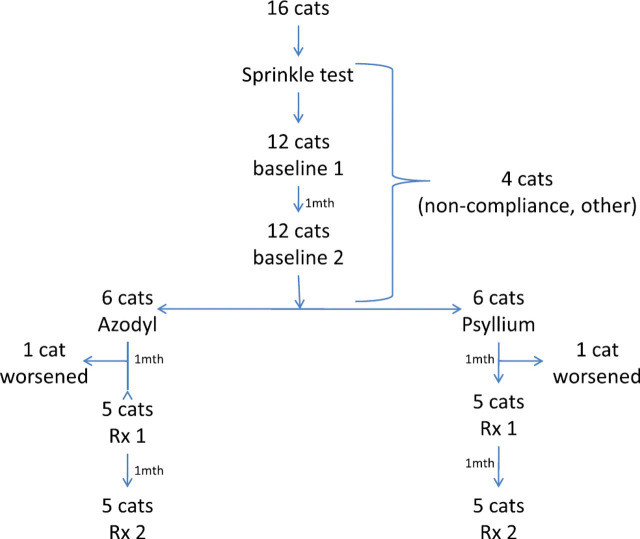 Study design diagram
Study design diagram
Dosage of Azodyl was determined based on the manufacturer’s guidelines, adjusted for the cat’s weight. Cats weighing under 5 lbs received one capsule daily, cats between 5 and 10 lbs received one capsule twice daily, and cats over 10 lbs received two capsules in the morning and one in the evening. Control capsules contained only psyllium husk and were identical in appearance to the Azodyl capsules.
Study Results: No Significant Improvement with Non-Recommended Azodyl Administration
The results of the study indicated that administering Azodyl by sprinkling it onto food or as a slurry did not lead to a significant reduction in azotemia in cats with CKD. There was no statistically significant difference in the percentage change of BUN or creatinine levels between the cats treated with Azodyl and those receiving the placebo (psyllium husk).
Table 1. BUN, Creatinine, and Albumin Concentrations in Cats Treated with Sprinkled Azodyl
| Group | Cat # | IRIS Stage | B1 (Baseline 1) | B2 (Baseline 2) | T1 (Treatment 1) | T2 (Treatment 2) | % Change BUN | % Change Creatinine | % Change Albumin |
|---|---|---|---|---|---|---|---|---|---|
| Placebo | C1 | 4 | 140 | 112 | 89 | 149 | -36.4 | -24.7 | -13.9 |
| Placebo | C2 | 3 | 51 | 53 | 68 | 56 | 5.7 | -18.2 | -2.9 |
| Placebo | C3 | 3 | 47 | 50 | 51 | 65 | 2 | -11.1 | -2.9 |
| Placebo | C4 | 2 | 38 | 40 | 39 | 53 | -2.5 | 3.8 | -8.6 |
| Placebo | C5 | 3 | 54 | 47 | 52 | 55 | -3.7 | 16.7 | -10.3 |
| Azodyl | A1 | 2 | 46 | 47 | 44 | 43 | -8.5 | 0 | -2.5 |
| Azodyl | A2 | 4 | 79 | 78 | 116 | 143 | 46.8 | 52.3 | 0 |
| Azodyl | A3 | 3 | 78 | 70 | 66 | 64 | -17.9 | -28.9 | -11.8 |
| Azodyl | A4 | 3 | 55 | 64 | 66 | 63 | -1.6 | -2.3 | -5.1 |
| Azodyl | A5 | 2 | 37 | 43 | 44 | 49 | 2.3 | -3.7 | -11.8 |
B1, B2 = baseline sample values; T1, T2 = treatment sample values. Bold numbers represent the baseline (maximum) and treatment (minimum) values used for % change calculation. IRIS = International Renal Interest Society Classification.
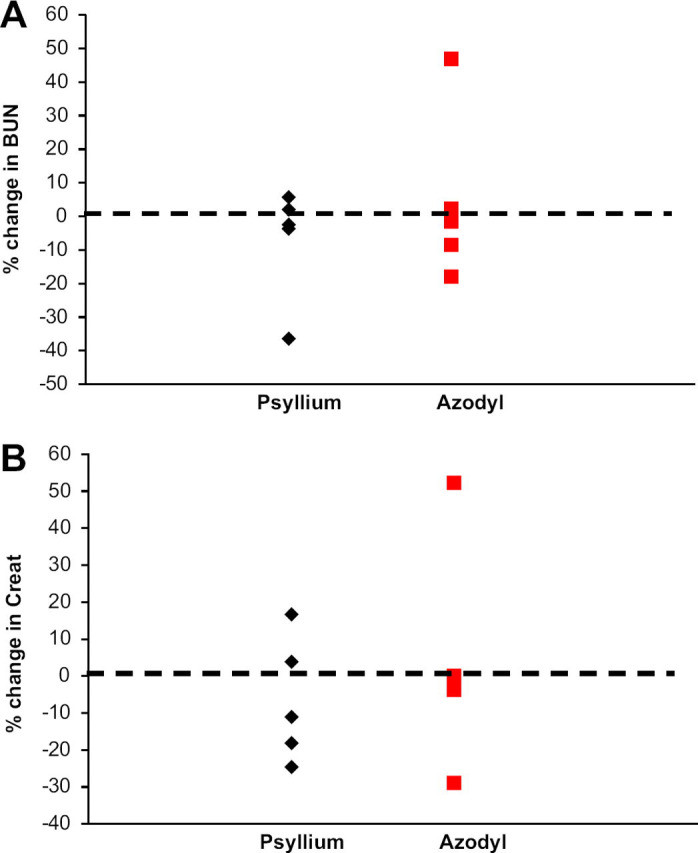 Percentage change in BUN and Creatinine
Percentage change in BUN and Creatinine
Albumin levels, which were monitored to account for potential dehydration affecting BUN levels, remained stable and within normal ranges for all cats throughout the study. This suggests that changes in azotemia were not influenced by hydration status.
Discussion: Implications for Azodyl Use in Cats with CKD
The findings of this study indicate that when Azodyl is administered by sprinkling the capsule contents onto food or mixing it into a slurry, it does not effectively reduce azotemia in cats with stable CKD. This outcome contrasts with some earlier studies suggesting potential benefits of synbiotics in reducing azotemia in other animal models and humans when administered as intended (intact capsules).
The discrepancy in results may be attributed to the method of administration. Sprinkling Azodyl onto food bypasses the enteric coating, potentially exposing the probiotic bacteria to degradation in the stomach’s acidic environment. Furthermore, Bifidobacterium longum, a key component of Azodyl, is an obligate anaerobe and may lose its efficacy upon exposure to air outside the capsule for a prolonged period before consumption.
While this study focused on the non-recommended administration method, it’s important to note that it does not address whether Azodyl is effective when given as intact capsules, as per the manufacturer’s recommendation. Further research is needed to investigate the efficacy of Azodyl when administered in the prescribed manner in cats with CKD.
The study acknowledges a previous uncontrolled case series that suggested some benefit from Azodyl sprinkled on food. However, the current study’s controlled design, including a placebo group and baseline stabilization period, provides a more rigorous assessment. The use of psyllium husk as a control is also relevant, as psyllium itself is a prebiotic and could theoretically have some effect on gut flora. However, the study’s findings align with previous research indicating that psyllium alone does not significantly reduce azotemia.
Conclusion: Administer Azodyl as Recommended for Cats
In conclusion, this study demonstrates that administering Azodyl by sprinkling capsule contents onto food or mixing it into a slurry is not effective in reducing azotemia in cats with stable CKD. Therefore, cat owners who choose to use Azodyl for their cats with CKD should be advised to follow the manufacturer’s recommended administration method – giving the capsules intact – to maximize the potential for any beneficial effects. Further research is warranted to determine if Azodyl, when given as intact capsules, can effectively reduce azotemia and improve outcomes for cats with chronic kidney disease. For now, based on this study, non-recommended administration methods should be avoided.
Acknowledgements: This study was funded by the VIN Foundation. Azodyl and control capsules were provided by Vetoquinol USA.
References: (Keep original references as in the source article)

