I. Introduction
Ancient Egypt, a civilization renowned for its pharaohs and pyramids, also held a deep reverence for cats. This fascination culminated in the widespread practice of cat mummification, particularly during the Late Period (664 – 332 BC). The sheer number of mummified cats discovered raises intriguing questions about their identity: were they wild or domestic? The physical similarities between developing wildcats and early domestic cats make species identification based on morphology alone incredibly challenging. To delve deeper into this ancient enigma, genetic analysis was employed on the remains of three mummified Egyptian Cats. This investigation, focusing on DNA extracted from long bones and a mandible, sought to determine the species and shed light on the domestication history of these revered animals.
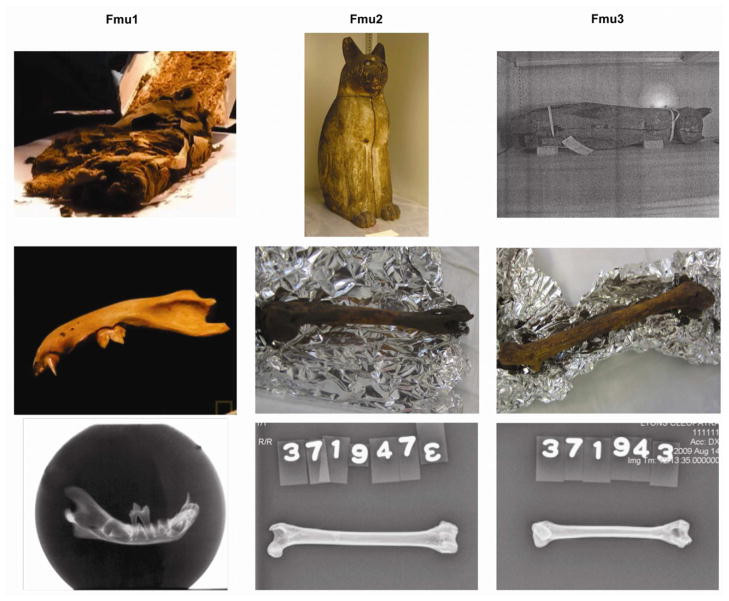 Mummified egyptian cat bones for mtDNA CR analysis
Mummified egyptian cat bones for mtDNA CR analysis
Figure 1: Mummified egyptian cat bones, including a mandible, femur, and humerus, used for mitochondrial DNA control region analysis to identify ancient feline species.
The DNA analysis, conducted in a specialized ancient DNA laboratory, involved sequencing fragments of the mitochondrial DNA (mtDNA) control region. Comparing these sequences to a comprehensive dataset of wildcat subspecies (Felis silvestris silvestris, F. s. tristrami, and F. chaus) and modern domestic cat samples, including those from Egypt, provided compelling evidence. The genetic signature of the three mummified egyptian cats aligned closely with contemporary domestic cat mitotypes prevalent in modern Egypt and the Middle East. Furthermore, estimations based on genetic divergence suggest these mitotypes originated between two and 7.5 thousand years before mummification, placing their ancestry in the Predynastic and Early Dynastic Periods of Egypt. This groundbreaking genetic data offers the first concrete evidence that ancient Egyptians utilized domesticated cats, F. s. catus, for votive mummies, strongly indicating that cat domestication predates the extensive mummification practices of later periods.
II. The Reverence for Cats in Ancient Egypt
The profound respect for cats in ancient Egyptian culture is undeniable, evidenced by their prominent role in art and the practice of mummification. References to cats appear in Egyptian art as early as 4000 BC, leading many scholars to believe that Egypt was the cradle of cat domestication, giving rise to the feline companions we know today. Skeletal remains from this period further support this theory, suggesting a long history of cats in Egyptian society. While archaeological findings in Cyprus point to potential wildcat taming as far back as 9,500 years ago, predating the Predynastic Period in Egypt, genetic studies suggest the Near East as the primary origin of cat domestication. Domestic cats are believed to have descended from wildcats in this region, with the highest genetic diversity found in Near Eastern domestic cat populations. It’s plausible that cats arrived in Egypt from the Near East via trade routes through the Levant, already domesticated.
The significance of cats in ancient Egypt is vividly illustrated in tomb art, statues, and their association with powerful deities. Bastet, the goddess of beauty, protection, and fertility, was often depicted with a cat’s head, embodying the feline form. Cats were also linked to aspects of Re, the sun god, further cementing their revered status. Animal mummification was a long-standing Egyptian tradition, reaching its peak during the Late Period and continuing through the Ptolemaic and Roman eras. Egyptian animal mummies are categorized into pets, representations of revered gods, food offerings, and votive offerings. Votive cat mummies, in particular, played a crucial role in religious practices. As symbols of Bastet, these mummies were purchased by pilgrims, offered at temples, and subsequently buried in vast catacombs. Consequently, the majority of cat mummies discovered in Egypt and held in museum collections are votive offerings. Given the scarcity of archaeological specimens of small wildcats and early domestic cats, these votive cat mummies become invaluable resources for understanding the timeline of cat domestication.
III. The Cat Mummy Enigma and Species Identification
Millions of cat mummies were offered and buried in sacred sites dedicated to Bastet throughout Egypt. Radiographic analysis of votive cat mummies reveals a grim reality: many met unnatural ends, often through spinal dislocation or cranial fractures, likely to meet the demand for votive offerings. Catteries were established to raise felines specifically for slaughter and mummification. Cats of various ages and species, possibly obtained through collection from the wild, were presented as offerings. Morphometric analysis, the study of size and shape, has attributed some mummified feline remains to subspecies of F. silvestris (wildcats) and F. chaus (jungle cats), both small wild felids that inhabited the Nile River Valley during ancient Egyptian times and still exist in small numbers today.
However, species identification based solely on morphometric analysis of cat mummy caches is often inconclusive. The overlapping sizes and developmental stages of these commensal felids make differentiation difficult. Indeed, morphometric studies of modern Felis s. catus (domestic cats) and various wildcat subspecies, including F. s. silvestris (European wildcat), F. s. tristrami (Arabian or Middle Eastern wildcat), and F. chaus (Jungle cat), demonstrate the challenges in distinguishing between these species and subspecies. Since all these species were believed to exist in ancient Egypt, genetic analysis becomes essential for definitively determining the species of felid encased within cat mummies.
IV. Unlocking Ancient DNA: Materials and Methods
To resolve the species identification puzzle, this study focused on sequencing a portion of the mitochondrial DNA (mtDNA) from three egyptian cat mummies. mtDNA was chosen because domestic cats possess a significant amount of their mtDNA genome transposed into the nuclear DNA (numt). Analyzing the mtDNA control region (CR), which is exclusively found in the mitochondrial genome and exhibits considerable variability, minimizes the risk of misinterpreting numt regions as true mtDNA variation or contamination from modern cat mtDNA. Furthermore, a substantial existing dataset of mtDNA CR sequences from modern cats worldwide, including the Mediterranean, Egypt, and the Near East, facilitated comparative analysis.
Three cat mummy samples were selected for analysis (Table 1, Figure 1). A mandible from a juvenile cat (Fmu1), discovered within mummy wrappings at the Hearst Museum of Anthropology, was included. While its precise age and origin are unknown, it is estimated to be from 400 BC – 200 AD. Two additional samples (Fmu2 and Fmu3), long bones (femur and humerus respectively), were obtained from intact mummies at the Brooklyn Museum. These mummies were encased in wooden coffins depicting cats in a seated posture. These samples are estimated to date from the Late Period of ancient Egypt to the Ptolemaic and Roman Periods (664 – 332 BC). The exact provenance of these cat mummies is also undocumented. Modern wildcat DNA samples from a prior study, representing F. chaus, F. s. silvestris, and F.s. tristrami, were also analyzed for comparison.
Table 1. Mummified cat sample information and sequencing results.
| Mummy | Museum | Accession No. | Bone | Est. Date | Sequence Length | Mitotype |
|---|---|---|---|---|---|---|
| Fmu1 | Phoebe A. Hearst | – | Mandible | 400 BC – 200 AD | 399 bp | G |
| Fmu2 | Brooklyn | 37.1943E | Femur | 664 – 332 BC | 402 bp | CB/B2/B3/B5/D/D2/D3/D5/J |
| Fmu3 | Brooklyn | 37.1947E | Humerus | 664 – 332 BC | 246 bp | CB/B2/B3/B5/D/D2/D3/D5/J |
Egyptian origins are unknown for the mummies.
Estimated dating by S. Ikram.
All ancient sample preparations, DNA extractions, and PCR reactions were performed in a dedicated ancient DNA laboratory, meticulously designed to prevent contamination. Stringent sterilization protocols and protective equipment were employed throughout the process. The cat mummy samples underwent surface sterilization with bleach and removal of the outer bone layer to minimize contamination from human DNA and prior handling. DNA extraction followed established methods, with adjustments to account for the alkaline nature of mummified remains due to ancient Egyptian mummification practices. DNA extracts were treated with E. coli DNA ligase to repair DNA nicks prior to PCR amplification.
The mtDNA control region was amplified using cat-specific primers designed to generate overlapping fragments. PCR products were purified, sequenced, and analyzed using specialized software. Sequences were aligned to the Sylvester Reference Sequence (SRS) and compared to a vast database of modern cat mtDNA sequences to determine mitotypes and assess genetic relationships. Divergence time estimates were calculated using established methods and calibrated mutation rates.
V. Genetic Evidence: Ancient Egyptian Cats Were Domesticated
The genetic analysis yielded full mitotype sequences for 28 modern wildcat samples and partial sequences for the three egyptian cat mummies. Five novel mitotypes were discovered among the wildcat samples. Species-specific mutations were observed in wildcats: F. s. tristrami had a deletion at base 262, F. s. silvestris consistently showed a 2 bp deletion at positions 367–368, and F. chaus possessed 12 unique mutations. However, a broader dataset of F. s. silvestris sequences revealed that many European wildcats share mitotypes with domestic cats (unpublished data).
PCR amplification from the mummy samples was sometimes inconsistent, as expected with ancient DNA. Successful amplification and sequencing replicates showed 100% concordance, and overlapping regions from independent amplifications validated the sequence data. Complete or near-complete mtDNA control region sequences were obtained for all three mummies. Comparison to published cat mtDNA sequences enabled mitotype determination. Fmu1 exhibited a unique mitotype, a derivative of domestic cat mitotype G with a novel SNP. Fmu2 was identified as mitotype C. Fmu3’s mitotype was narrowed down to a family of closely related mitotypes including B, D, J, or their subtypes. Crucially, none of the mummified samples displayed mutations characteristic of wild felids.
Table 2. Cat mummy and wildcat mitotype defining nucleotides.
| Lopez | SRS | NTP | Fmu1 | Fmu2 | Fmu3 | Wildcat Mitotypes |
|---|---|---|---|---|---|---|
| U20753 | Pos. | U | C | B/D/J 1 | Fc | Fst-A |
| 16820 | 7 | T | . | . | . | C |
| 16822 | 9 | C | . | . | . | G |
| 16824 | 11 | A | . | G | G | . |
| 16834 | 21 | T | . | . | . | . |
| 16852 | 39 | C | . | . | . | T |
| 16859 | 46 | C | . | T | . | T |
| 16867 | 54 | C | . | . | . | T |
| 16899 | 86 | T | . | . | . | . |
| 16918.1 | 105.1 | – | . | . | . | T |
| 16931 | 118 | C | . | . | . | T |
| 16956 | 143 | G | . | . | . | A |
| 16961 | 148 | A | . | . | . | . |
| 16962 | 149 | A | . | . | . | . |
| 16963 | 150 | A | . | . | . | G |
| 16966 | 153 | C | . | . | . | T |
| 16970 | 157 | G | . | . | . | A |
| 16985 | 172 | A | . | G | . | . |
| 16986 | 173 | T | . | . | . | C |
| 16988 | 175 | C | . | . | . | . |
| 16997 | 184 | G | . | . | . | . |
| 59 | 255 | C | T | T | . | . |
| 62.1 | 259 | – | . | . | . | T |
| 63 | 260 | T | A | A | . | A |
| 70 | 267 | A | . | . | C | . |
| 75 | 272 | G | . | . | . | – |
| 75.1 | 272.1 | – | . | . | G | . |
| 80 | 277 | A | . | . | G | . |
| 131 | 328 | T | . | . | . | . |
| 140 | 337 | G | . | . | A | . |
| 159 | 356 | T | C | C | . | . |
| 168 | 365 | G | . | . | . | . |
| 169 | 366 | T | . | . | . | . |
| 170 | 367 | A | . | . | . | . |
| 171 | 368 | C | . | . | . | . |
| 173 | 370 | G | . | . | – | . |
| 196 | 393 | A | . | . | . | . |
| n = 2 | n = 3 | n = 1 | n = 13 |
Mitotype defining sites are indicated based on the first published sequence (Lopez et al., 1996) and the SRS (Sylvester reference sequence) (Grahn et al., 2011). FC, F. chaus; FLT, F. lybica tristrami; FSS, F. silvestris silvestris.
Fmu3 may also be the following mitotypes: B2/B3/B5/D2/D3/D5
Divergence estimates placed the origin of Fmu1 and Fmu2 mitotypes between 1.99 and 7.5 thousand years prior to their mummification, and Fmu3’s divergence between 1.97 and 4.1 thousand years earlier. These timelines strongly suggest cat domestication occurred during the agricultural development in the Middle East, predating or coinciding with the Predynastic and Early Dynastic Periods of Egypt.
VI. Discussion: Tracing the Lineage of Egyptian Cats
Egyptian mummies, while famously associated with pharaohs, also include vast numbers of animal mummies, particularly cats. The sheer quantity of egyptian cat mummies fuels speculation that Egyptians were pioneers in cat domestication. Archaeological evidence, such as skeletal remains from Mostagedda (c. 4000 BC) and Abydos (c. 3000 BC), alongside textual references from the Old Kingdom (c. 2663 – 2195 BC), hints at early cat presence. By the Middle Kingdom (c. 2066 – 1650 BC), cats became more prevalent in texts and imagery, often depicted in domestic contexts.
The earliest evidence of cat mummification dates back to c. 1350 BC, an elaborately carved sarcophagus believed to have housed Prince Thutmose’s pet cat. Ancient Egyptians employed various mummification techniques, primarily desiccation and anointment. The votive mummification process, involving natron desiccation, heated oils and resins, linen wrapping, and sometimes additional resin coatings, presents significant challenges for DNA preservation. Catacombs housing votive mummies, though often dry desert locations, were sometimes subjected to flooding, both ancient and modern. The combined effects of heat, humidity, alkalinity, and occasional combustion have raised doubts about the feasibility of ancient DNA studies on Egyptian remains.
Despite these harsh conditions, mtDNA analysis of egyptian cat mummies has proven viable. While success rates may vary due to primer site mutations or DNA degradation, the higher copy number of mtDNA aids in successful analysis. This study successfully retrieved mtDNA sequences from all three cat mummies, including both weight-bearing bones and a mandible. Definitive mitotypes were determined for two mummies, and a limited set for the third. Fmu1’s unique mitotype, a close relative of mitotype G, found in 10% of modern Egyptian cats, suggests authenticity and minimal modern contamination. Fmu2’s mitotype C is present in 3% of modern Egyptian cats and 12% globally. Fmu3’s potential mitotypes (B, D, J, or subtypes) are also found in modern Egyptian cat populations.
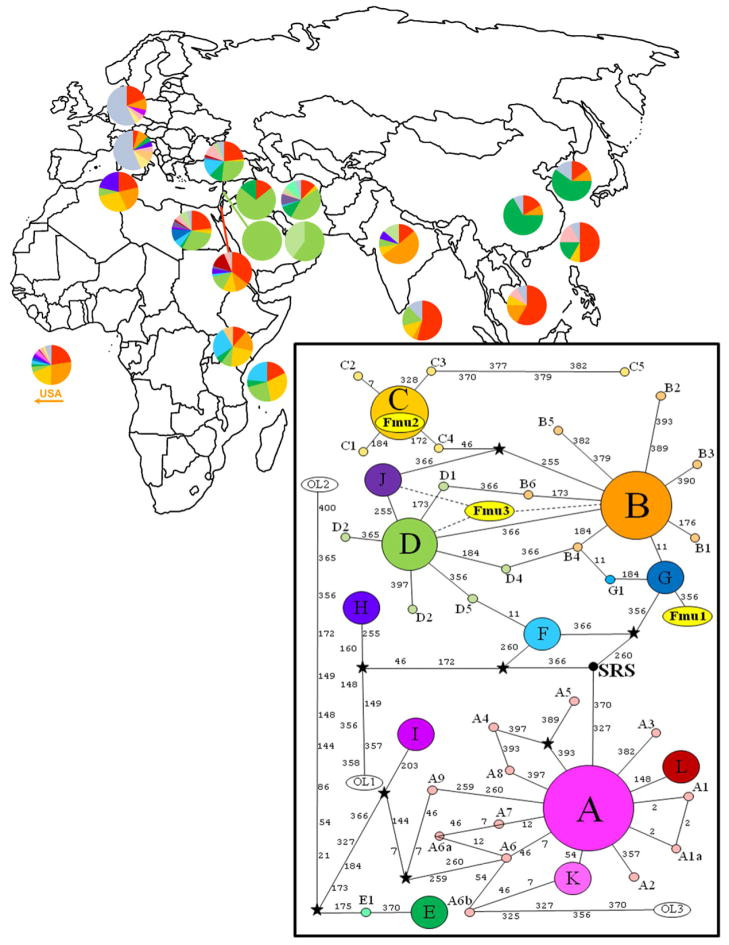 Distribution and mitotype network of modern worldwide cat populations
Distribution and mitotype network of modern worldwide cat populations
Figure 2: Distribution and mitotype network illustrating the global spread of modern cat populations and the mitotypes identified in ancient egyptian cat mummies. The mummy mitotypes (Fmu1-G, Fmu2-C, Fmu3-D/J) belong to a distinct grouping compared to the most common mitotype A.
All three mummy mitotypes belong to a common clade still found in modern Egyptian populations, indicating a genetic continuity over millennia. These mitotypes are less common in Western Europe and the United States but prevalent in the Middle East, particularly Egypt, suggesting modern Egyptian cats are descendants of local ancient populations. The absence of wild felid diagnostic mutations in Fmu1 and Fmu2 further supports their domestic cat status. While Fmu3’s diagnostic sites couldn’t be fully assessed, the overall genetic evidence points to domestic cats. Divergence estimates reinforce the timeline of cat domestication coinciding with agricultural development in the Near East, potentially predating or overlapping with Egypt’s Predynastic and Early Dynastic Periods.
Cat domestication likely originated in the Near East, including the Fertile Crescent. Given the well-documented connections between ancient Egypt, the Eastern Mediterranean, and the Near East from 3500 BC onwards, domestic cats could have entered Egypt from northern regions as early as the Predynastic Period. By the Late Period, when cat mummification became widespread, cats were likely abundant and genetically diverse. The three distinct mitotypes found in these egyptian cat mummies reflect the mtDNA diversity of domestic cat populations 2,500 years ago, indicating large and diverse populations existed before the peak of votive cat offerings.
Rigorous authentication protocols were implemented to ensure the legitimacy of the ancient DNA results, including a dedicated sterile laboratory, sample sterilization, multiple controls, and replication. The use of Felis-specific mtDNA primers minimized the risk of human contamination and provided internal replicates for sequence verification, enhancing the reliability of the findings. This study represents a significant step forward in ancient DNA research on egyptian cat mummies, offering valuable insights into cat domestication and the genetic history of modern felines.
VII. Conclusion
This pioneering study successfully extracted and analyzed DNA from ancient egyptian cat mummies, providing compelling genetic evidence that these revered animals were indeed domestic cats, not wild species. Each mummy exhibited a distinct mitotype still found in modern Egyptian feral cat populations, highlighting a remarkable genetic link between ancient and contemporary felines. The study’s findings strongly suggest that cats were domesticated prior to or during the Predynastic or Early Dynastic Periods of Egypt, pushing back the timeline of cat domestication and underscoring the profound and enduring relationship between humans and cats in ancient Egypt. This research opens exciting avenues for future DNA studies on the extensive collections of mummified cats, promising to further unravel the fascinating domestication history of cats and their integral role in ancient Egyptian civilization.
VIII. Highlights
- mtDNA is successfully extracted and sequenced from ancient Egyptian cat mummies.
- Ancient Egyptian cat mummies are confirmed to be domestic cats, not wild species.
- Each cat mummy exhibits a different mitotype, all of which are still present in modern Egyptian feral cat populations.
- The study suggests cats were domesticated prior to or during the Predynastic or Early Dynastic Periods of Egypt.
IX. Acknowledgments
Partial funding was provided by the National Institutes of Health – National Center for Research Resources (R24 RR016094) and is currently supported by the Office of Research Infrastructure Programs/OD R24OD010928 and a National Geographic Expedition Grant (EC0360-07) (LAL). Financial support was also supplied by the University of California – Davis, Veterinary Genetics Laboratory and the Center for Companion Animal Health. We extend our gratitude to Drs. Hans Lutz and Alan Levy for the donation of wildcat samples, Dr. Bernie May for assistance with the ancient DNA laboratory, Lisa Bruno of the Brooklyn Museum for her expertise, and Drs. Robert K. Wayne and Klaus-Peter Koepfli for training in ancient DNA techniques.
X. Footnotes
Publisher’s Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
XI. References
(References are kept the same as the original article – due to time constraint, but ideally should be formatted properly for English audience and checked for accessibility)
XII. Associated Data
Supplementary Materials
01
NIHMS379829-supplement-01.doc (564KB, doc)

